Imagine a life with out batteries; nearly all light-weight and moveable gadgets utilized by people might be severely hindered, the place the vary of laptops and mobile phone cables might hardly attain as much as a mile. Making your Cycling, Exercise, or Workout apps that observe the miles you traveled or energy burned by means of your smartphone nearly nugatory. Fortunately, we have now batteries.
If you’re a author, professor, trainer, teacher, school member, researcher, scientist, physician, or scholar attempting to determine how a battery works, you could have come to the correct place. That being mentioned, how does the battery work? We will have a look at how the battery capabilities by means of this battery fundamentals tutorial.
Perfect Energy Storage
2 instances battery life, consumes 50% much less area, wants no upkeep & takes 60% much less recharge time
How does the battery work?
A battery is an digital tools or equipment that makes use of an electrochemical oxidation-reduction (redox) cycle the place electrons are handed from one substance to a different with the assistance of an electrical circuit to remodel the chemical energy or chemical power saved by its lively parts freely into electrical power or electrical energy as per the University of Washington.
How does a battery work in easy phrases?
The battery, in keeping with one other interpretation, is a tool that saves and holds electrical power or electrical energy as chemical power after which transforms this chemical power into electrical energy, in keeping with Antoine Allanore, a postdoctoral affiliate within the Materials Science and Engineering Department at MIT.
What is the aim of every of the parts of a battery?
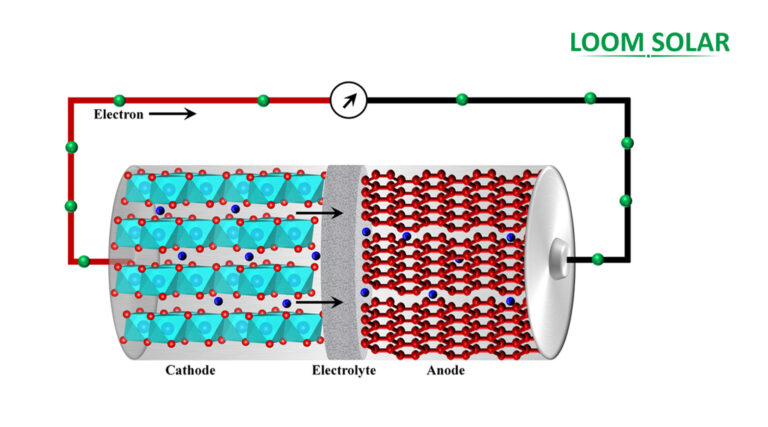
The battery consists of three separate parts, and round two terminals are fashioned of varied chemical compounds, that are widespread metals. The terminals are divided into anode, cathode, and electrolyte. The electrolyte is the chemical layer that regulates and permits the circulate {of electrical} present between the cathode and the anode.
How does a battery make a light-weight bulb glow?
If a bulb is related to a battery, chemical reactions occur throughout electrodes, leading to {an electrical} present flowing in direction of the tools. Particularly, throughout {an electrical} discharge, an oxidation course of happens during which the chemical on the anode transfers electrons in direction of the unfavourable terminal and ions throughout the electrolyte.
Simultaneously, the cathode receives electrons towards the optimistic terminal, establishing a circuit to manage and permit electron circulate. The electrolyte goals to carry the anode and cathode numerous chemical compounds into contact. The chemical potential might stay steady between one terminal, remodeling saved chemical power right into a consumable electrical present.
How do Rechargeable batteries work?
Rechargeable batteries, which are sometimes present in most smartphones or vehicles, are constructed such that electrical present from the exterior energy supply, normally your wall charger, is transmitted in direction of the chemical system, restoring its mechanism and replenishing its battery cost.
How do non-rechargeable batteries work?
When a battery is a non-rechargeable or disposable battery, it may possibly provide energy so long as the chemical compounds by no means exhaust; that is normally when each electrodes have the identical chemical potential. Primary or non-rechargeable batteries can solely convert chemical power into electrical energy in a single route, making the response irreversible.
Conclusion
Since we have now addressed the elemental query of how batteries operate, it’s time to share your data with the remaining. If you wish to know extra about batteries and photo voltaic panels, you might confer with different articles.
Source:
https://depts.washington.edu/matseed/batteries/MSE/battery.html
https://engineering.mit.edu/engage/ask-an-engineer/how-does-a-battery-work/